icobrain dm
Supporting differential diagnosis and monitoring of atrophy in dementia
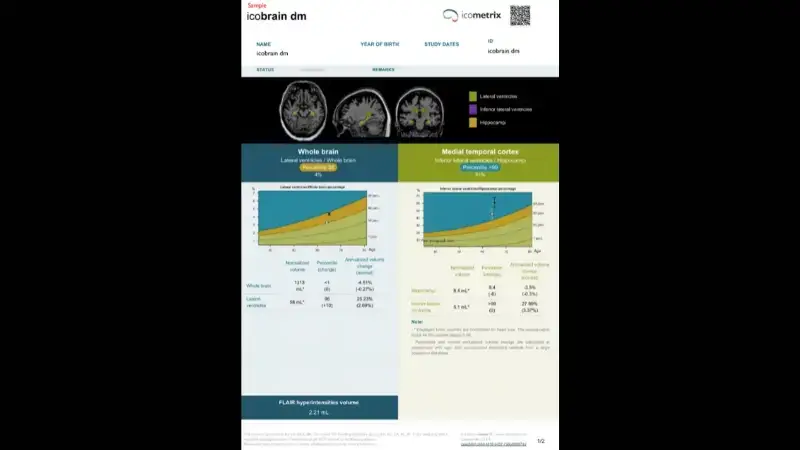
icobrain dm is designed to support the early detection of markers of dementias, comparing whole brain and ventricular volumes to age- and sex-matched healthy controls. icobrain dm can also used to assist radiologists and neurologists with the differential diagnosis of the particular type of dementia a patient may have, by providing individual volumes of the cortical lobes and hippocampi, and reporting percentiles compared to the age- and sex-matched healthy control population. Additionally, icobrain dm can help radiologists and neurologists to evaluate eligibility for amyloid-targeting treatments for Alzheimer's disease, by reporting out the total volume of FLAIR white matter hyperintensities, as well as the number of T2* GRE hypointensities.
Automatic analysis of 3D T1 sequences
Enables early detection, follow-up and differential diagnosis of Alzheimer and other dementia
Quantifies and tracks cortical brain volumes and asymmetries to differentiate common dementia types (frontal, parietal, temporal, occipital, hippocampal)
Comparing volumes and volume changes to an age-and sex- matched normative reference population
40% Reduction in radiological reading time
35% increased radiological consistency
Automated standardized reports
Save time, secure your diagnosis and optimize your workflow with Incepto
Publications
-
Van Hecke W, Costers L, Descamps A, Ribbens A, Nagels G, Smeets D, Sima DM. A Novel Digital Care Management Platform to Monitor Clinical and Subclinical Disease Activity in Multiple Sclerosis. Brain Sci. 2021 Sep 3;11(9):1171. doi: 10.3390/brainsci11091171. PMID: 34573193; PMCID: PMC8469941
-
Sima DM, Wilms G, Vande Vyvere T, Van Hecke W, Smeets D. On the use of icobrain's prepopulated radiology reporting template for multiple sclerosis follow-up. ECR 2020 / C-11342
-
Calloni, S.F., Vezzulli, P.Q., Castellano, A. et al. Combining semi-quantitative rating and automated brain volumetry in MRI evaluation of patients with probable behavioural variant of fronto-temporal dementia: an added value for clinical practise?. Neuroradiology 65, 1025–1035 (2023). https://doi.org/10.1007/s00234-023-03133-w
-
Hanne Struyfs, Diana Maria Sima, Melissa Wittens, Annemie Ribbens, Nuno Pedrosa de Barros, Thanh Vân Phan, Maria Ines Ferraz Meyer, Lene Claes, Ellis Niemantsverdriet, Sebastiaan Engelborghs, Wim Van Hecke, Dirk Smeets. Automated MRI volumetry as a diagnostic tool for Alzheimer's disease: Validation of icobrain dm. NeuroImage: Clinical, Volume 26, 2020, 102243,
ISSN 2213-1582, https://doi.org/10.1016/j.nicl.2020.102243.
Regulatory
Contact us to know if this product is available in your country.

