icobrain ep
Supporting differential diagnosis of epilepsy through abnormality patterns
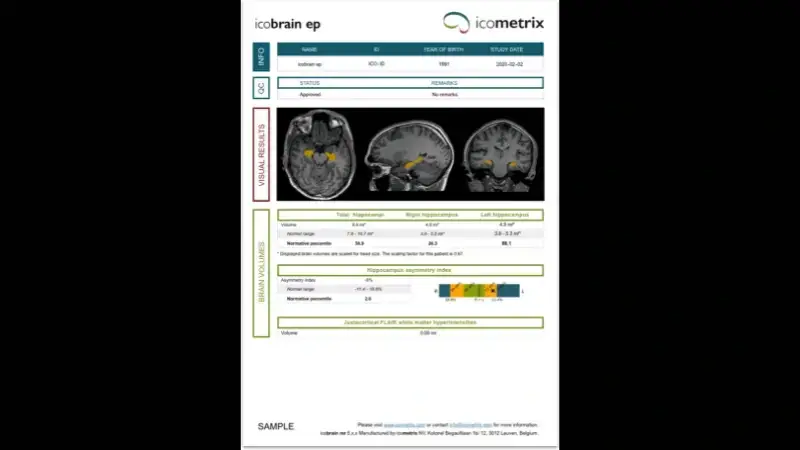
icobrain ep is used as part of the assessment of mesial temporal lobe epilepsy, reporting lateralized hippocampal volumes and their percentiles, as well as the hippocampal asymmetry index. If an optional FLAIR scan is provided, the icobrain ep report also evaluates FLAIR hyperintensities adjacent to the cortex, which may be indicative of cortical dysplasia.
Uncovers abnormality patterns
Compare hippocampal asymmetry to an age-and sex matched normative healthy population
Assess juxtacortical abnormalities indicative of cortical malformations
Compare volumes and volume changes to an age-and sex- matched normative reference population.
Augment & speed up radiology reporting
Enhance sensitivity
Save time, secure your diagnosis and optimize your workflow with Incepto
Publications
No items found
Regulatory
icobrain ep is a report of icobrain mr. icobrain mr medical device is a regulated health product that bears the CE mark, Class IIa. Notified Body: SGS Belgium NV. n°1639. Manufacturer: Icometrix NV.
icobrain mr is a diagnostic tool and shall not be used alone to recommend medical care for diagnostic purposes. Please read the instructions in the user manual carefully. Please contact contact@incepto-medical.com for more information. Last update: 2025/06/03
Contact us to know if this product is available in your country.

