KEROS
AI solution for diagnostic assistance for knee MRI
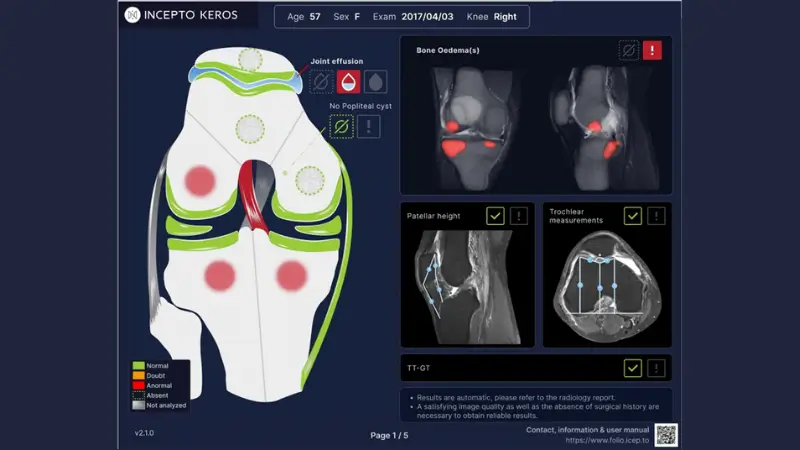
KEROS is an AI solution for knee MRI diagnosis. KEROS automatically analyzes the primary knee anatomical structures and detects ACL tears, MCL tears, meniscal tears, ligament, menisci, and cartilage lesions along with identifying bone edema, joint effusions, and popliteal cysts. And KEROS automatically measures patellar height, trochlear dysplasia, and TT-TG and provides comparison to reference values.
Lesion detection and characterization
KEROS detects and characterizes ACL, MCL, menisci and cartilages lesions as well as joint effusion, popliteal cyst and bone edema. Bone edema is segmented and represented on a 3D rendering view.
Automatic patellar instability characterization
KEROS provides Insall-Salvati and Caton-Deschamps ratios, sulcal depth, sulcus angle, facet asymmetry ratio, TT-TG, and compares them to MRI reference values.
Automatic pre-filled structured report generation
KEROS automatically generates a schematic report in SCPT format. When integrated with the reporting software, the radiologist’s report can even be automatically pre-filled with KEROS’s findings and finalized in the RIS. With Incepto's reporting solution, Polaris, radiologists can edit the pre-filled structured report and upload the revised report directly to the RIS.
Saves time on exam intrepretation
Improves user performance
Structure communication with patients and physicians
Save time, secure your diagnosis and optimize your workflow with Incepto
Publications
-
Meniscal lesion detection and characterization in adult knee MRI: a deep learning model approach with external validation, in Physica Medica (2021)
-
Deep learning to detect anterior cruciate ligament tear on knee MRI: first multi-continental external validation, in European Radiology (2022)
-
Impact of AI for pathology detection from knee MRI , Abstract RSNA (2022)
-
Comparative performance of an artificial intelligence algorithm and human annotators for meniscal tear detection, Abstract JFR (2022)
-
Performance of automatic patella alta detection in MRI using deep learning: validation, Abstract JFR (2024)
-
Accuracy for Bone Marrow Lesions detection in knee MRI using a fully-automated AI algorithm, Abstract ECR (2024)
Regulatory
Contact us to know if this product is available in your country.

